Embark on an enlightening journey with our atomic spectra lab answer key, a comprehensive guide that unravels the mysteries of light and energy. Discover the principles of spectroscopy, the secrets of spectral line identification, and the fascinating applications of atomic spectra in various scientific disciplines.
Prepare to delve into the captivating world of atomic spectra, where the dance of electrons reveals the inner workings of atoms. Our meticulously crafted answer key will illuminate your path, empowering you to decipher the language of light and uncover the secrets of the universe.
Atomic Spectra Lab Overview

The atomic spectra lab is designed to provide students with a hands-on experience in the study of atomic spectra. The objectives of the lab are to:
- Observe the emission spectra of various elements
- Identify the elements present in a sample based on their emission spectra
- Understand the relationship between the energy levels of atoms and the wavelengths of light they emit
The equipment and materials used in the lab include:
- A spectroscope
- A Bunsen burner
- A variety of metal salts
The step-by-step procedure for conducting the lab experiment is as follows:
- Set up the spectroscope according to the manufacturer’s instructions.
- Light the Bunsen burner.
- Dip a clean wire loop into a sample of a metal salt.
- Hold the wire loop in the flame of the Bunsen burner.
- Observe the emission spectrum of the metal salt through the spectroscope.
- Record the wavelengths of the lines in the emission spectrum.
- Identify the element present in the sample based on the wavelengths of the lines in the emission spectrum.
Spectroscope Analysis
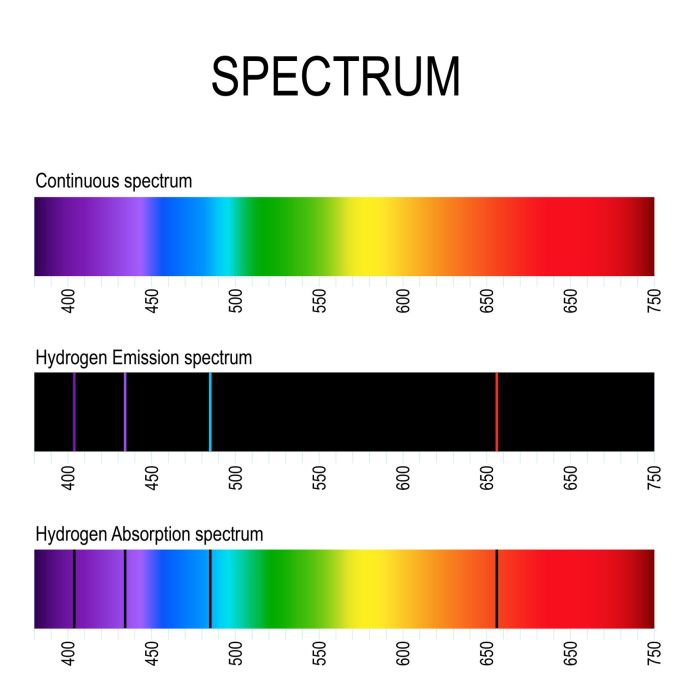
Spectroscopy is the study of the absorption and emission of electromagnetic radiation by matter. It is used to analyze the composition and structure of atoms, molecules, and ions. Spectroscopes are instruments used to measure the wavelengths of electromagnetic radiation emitted or absorbed by a sample.
Atomic spectra lab answer keys can be helpful in understanding the complex patterns of light emitted by atoms. If you’re looking for a comprehensive review of U.S. government and politics, check out the ap gov ultimate review packet . It covers everything from the Constitution to the current political landscape.
Returning to the topic of atomic spectra, the answer key can also provide insights into the structure and properties of atoms.
Types of Spectroscopes
There are many different types of spectroscopes, each with its own advantages and disadvantages. Some of the most common types of spectroscopes include:
- Prism spectroscopesuse a prism to disperse light into its component wavelengths.
- Grating spectroscopesuse a diffraction grating to disperse light into its component wavelengths.
- Interferometer spectroscopesuse interference to measure the wavelengths of light.
Using a Spectroscope, Atomic spectra lab answer key
To use a spectroscope, the sample is placed in the light path of the instrument. The light from the sample is then dispersed into its component wavelengths, and the wavelengths are measured. The resulting spectrum can be used to identify the elements present in the sample and to measure their concentrations.
Spectral Line Identification

Spectral line identification is a technique used to determine the elemental composition of a sample by analyzing the wavelengths of light it emits or absorbs. Each element has a unique set of spectral lines, which are caused by the absorption or emission of photons as electrons transition between energy levels within the atom.
The wavelength of a spectral line is inversely proportional to the energy difference between the two energy levels involved in the transition. The higher the energy difference, the shorter the wavelength of the spectral line.
Spectral Line Wavelengths and Energy Levels
The following table lists the wavelengths of some common spectral lines and their corresponding energy levels:
| Element | Wavelength (nm) | Energy Level (eV) |
|---|---|---|
| Hydrogen | 656.3 | 1.89 |
| Helium | 587.6 | 2.11 |
| Sodium | 589.0 | 2.10 |
| Potassium | 766.5 | 1.62 |
| Calcium | 422.7 | 2.93 |
Rydberg Formula
The Rydberg formula can be used to calculate the energy levels of atoms. The formula is:
E =
13.6 eV / (n^2)
where E is the energy of the atom in electron volts (eV) and n is the principal quantum number of the energy level.
Applications of Atomic Spectra

Atomic spectra have numerous applications in various scientific fields, including astronomy, chemistry, and medicine. They provide valuable insights into the composition and properties of matter.
Astronomy
In astronomy, atomic spectra are used to determine the composition of stars and other celestial objects. By analyzing the wavelengths and intensities of spectral lines, astronomers can identify the elements present in these objects. This information helps us understand the chemical evolution of the universe and the processes that occur within stars.
Analytical Chemistry
In analytical chemistry, atomic spectra are used to identify and quantify elements in a sample. Techniques like atomic absorption spectroscopy and atomic emission spectroscopy are commonly employed to determine the concentration of specific elements in various matrices, such as environmental samples, food products, and biological fluids.
Medical Diagnostics
In medicine, atomic spectra find applications in various diagnostic techniques. For example, in flame photometry, the emission spectra of elements like sodium and potassium are used to measure their concentrations in bodily fluids, aiding in the diagnosis and monitoring of electrolyte imbalances.
Popular Questions: Atomic Spectra Lab Answer Key
What is the purpose of an atomic spectra lab?
Atomic spectra labs provide hands-on experience in analyzing the light emitted by atoms, allowing students to understand the principles of spectroscopy and the relationship between the wavelength of light and the energy levels of atoms.
How does a spectroscope work?
A spectroscope separates light into its component wavelengths, allowing us to identify the specific wavelengths of light emitted by atoms. This information can then be used to determine the energy levels of the atoms.
What is the Rydberg formula?
The Rydberg formula is a mathematical equation that can be used to calculate the wavelengths of light emitted by atoms. It is named after the Swedish physicist Johannes Rydberg, who first developed the formula in the late 19th century.