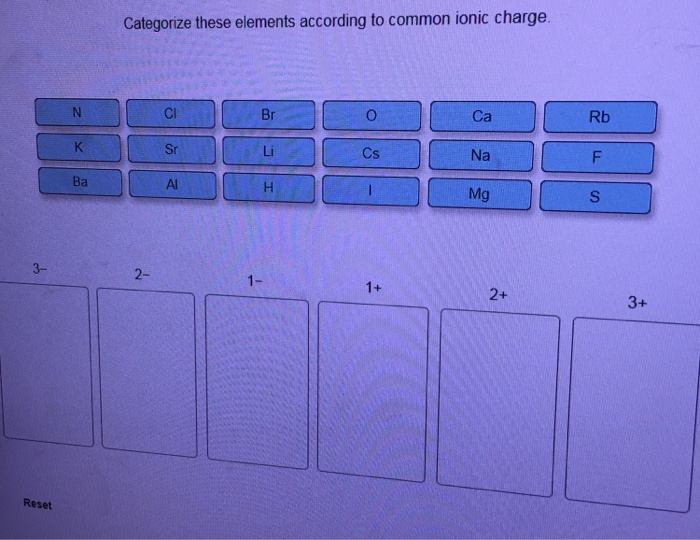
Categorize these elements according to common ionic charge. – Categorizing elements according to their common ionic charge is a crucial aspect of understanding their chemical behavior and properties. This comprehensive guide delves into the concept of ionic charge, exploring the factors that influence it and the various methods used for categorization.
With real-world examples and practical applications, this exploration unravels the significance of ionic charge in diverse fields, including chemistry, materials science, and medicine.
1. Introduction
Ionic charge is the electrical charge carried by an ion, which is an atom or molecule that has lost or gained electrons. Categorizing elements according to their ionic charge is important because it provides insights into their chemical properties and behavior.
For example, elements with a positive ionic charge (cations) tend to form ionic bonds with elements with a negative ionic charge (anions). The ionic charge of an element is determined by the number of electrons it has gained or lost.
2. Common Ionic Charges
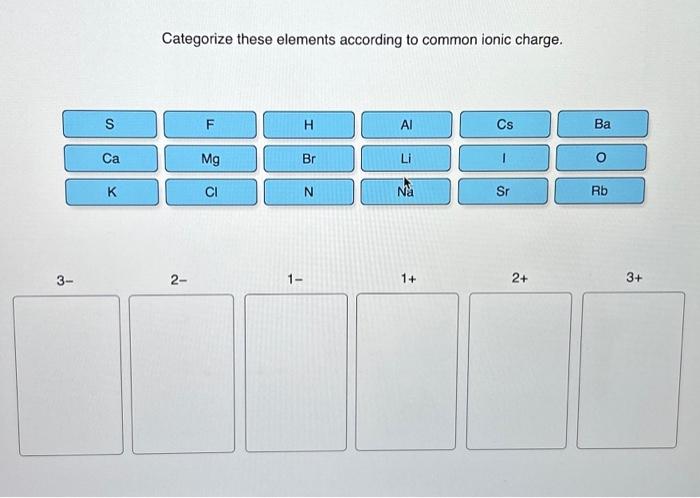
The most common ionic charges exhibited by elements are:
- +1 (cations)
- -1 (anions)
- +2 (cations)
- -2 (anions)
The factors that influence the ionic charge of an element include its atomic number, electron configuration, and electronegativity.
3. Methods for Categorizing Elements
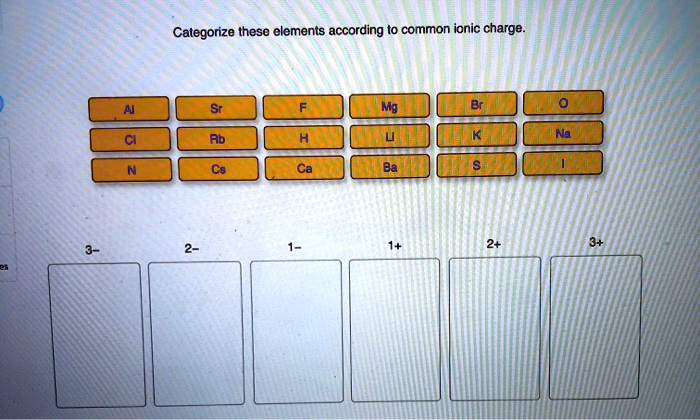
There are various methods used to categorize elements based on their ionic charge, including:
- The periodic table: Elements can be categorized into groups based on their ionic charge. For example, Group 1 elements (alkali metals) typically have a +1 ionic charge, while Group 17 elements (halogens) typically have a -1 ionic charge.
- The oxidation state: The oxidation state of an element is a measure of its ionic charge. Elements with a positive oxidation state have lost electrons, while elements with a negative oxidation state have gained electrons.
4. Examples of Categorization: Categorize These Elements According To Common Ionic Charge.
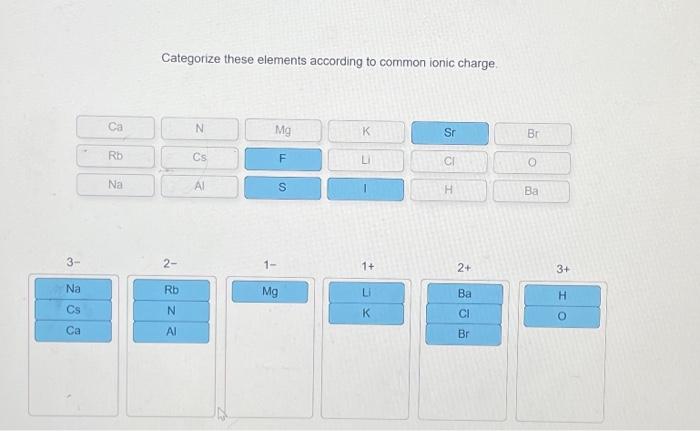
The following table shows examples of how elements can be categorized according to their ionic charge:
| Element | Ionic Charge |
|---|---|
| Sodium | +1 |
| Chloride | -1 |
| Magnesium | +2 |
| Oxide | -2 |
5. Applications of Categorization
Categorizing elements based on their ionic charge has practical applications in various fields, including:
- Chemistry: Ionic charge is used to predict the chemical properties of elements and to design new materials.
- Materials science: Ionic charge is used to design materials with specific properties, such as conductivity and strength.
- Medicine: Ionic charge is used to develop drugs and to understand the interactions between ions and biological molecules.
Q&A
What is ionic charge?
Ionic charge refers to the electrical charge carried by an atom or molecule that has lost or gained electrons, resulting in a net positive or negative charge.
Why is it important to categorize elements based on ionic charge?
Categorizing elements based on ionic charge helps predict their chemical behavior, reactivity, and interactions with other elements, enabling researchers to understand and manipulate their properties for various applications.